In India, it has emerged that a
Novartis drug,
diclofenac, administered to cattle as a painkiller since the 1990s (used for lameness, mastitis, and other common problems), is leading to serious depletion in the populations of
Indian vultures. The drug is a common human anti-inflammatory and, as such, a ban on its veterinary use has been resiste
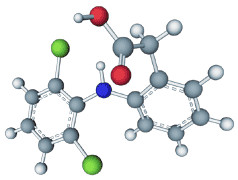
d to date for fear that this would decimate public trust in (and thus the market for) its use for humans. According to
The Guardian report, the Indian market makes up 90% of the Asian market for diclofenac.
In
earlier reports, the declines were attributed to a number of possible factors, including an unidentified virus, however the problem of
diclofenac toxicity in vultures was discovered in
2004. Nevertheless, the drug has not been withdrawn from its veterinary use, although the Indian government announced in March 2005 that phasing out of the drug would be imposed. The problem is caused by the administration of the drug to animals close to death. The drug is then present in the carcasses upon which the vultures feed. It has been estimated that over 99% of Indian vultures may have suffered poisoning. Losses are also being experienced in Nepal and Pakistan.
A new report published in the
Public Library of Science (PLoS) journal,
Biology (an open access publication) claims to have found an alternative in the drug
meloxicam. It is hoped that the effectiveness of this drug will overcome the reliance on diclofenac, despite the obstacles faced thus far to the complete phasing out of the drug.
The depletion in vulture populations has also had an impact on local traditional methods of disposing of the dead by sky burials, where vultures pick the bodies clean on what are known as
towers of silence. Without adequate populations, such methods are being abandoned in favour of alternatives, such as cremation. The crisis has emerged as one of both biological and cultural cultural diversity.